Oct 15, 2020
University of Minnesota – In the largest clinical trial of its kind, researchers show that combining sound and electrical stimulation of the tongue can significantly reduce tinnitus, commonly described as “ringing in the ears.”
They also found that therapeutic effects can be sustained for up to 12 months post-treatment.
The findings could potentially help millions of people since tinnitus affects about 10 to 15 percent of the population worldwide.
The study was conducted by researchers from the University of Minnesota, Trinity College, St. James’s Hospital, University of Regensburg, University of Nottingham, and Irish medical device company Neuromod Devices Limited.
The research was published as the cover story of Science Translational Medicine, an interdisciplinary medical journal by the American Association for the Advancement of Science (AAAS).
University of Minnesota Associate Professor Hubert Lim in the Department of Biomedical Engineering (College of Science and Engineering) and the Department of Otolaryngology (Medical School) was the senior author of the study sponsored by Neuromod Devices. Lim also serves as chief scientific officer of Neuromod Devices.
The study represents the largest and longest followed-up clinical trial ever conducted in the tinnitus field for a medical device with 326 enrolled participants, providing evidence regarding the safety, efficacy, and patient tolerability of bimodal neuromodulation for the treatment of tinnitus.
About 86 percent of treatment compliant participants reported an improvement in tinnitus symptom severity when evaluated after 12 weeks of treatment, with many experiencing sustained benefit 12 months post-treatment.
Lim said:
“I am truly proud of our company’s ability to perform such a large-scale randomized clinical trial in two countries.
“This study tracked the post-treatment therapeutic effects for 12 months, which is a first for the tinnitus field in evaluating the long-term outcomes of a medical device approach. The outcomes are very exciting and I look forward to continuing our work to develop a bimodal neuromodulation treatment to help as many tinnitus sufferers as possible.”
The study was conducted at the Wellcome Trust-HRB Clinical Research Facility, St. James’s Hospital, Dublin, Ireland, and the Tinnituszentrum of the University of Regensburg, Germany.
There were consistent therapeutic outcomes across both clinical sites, with no serious adverse events. The Minneapolis-based branch of NAMSA, the world’s only medical research organization, guided and assisted the close-out process of Neuromod Device’s clinical trial.
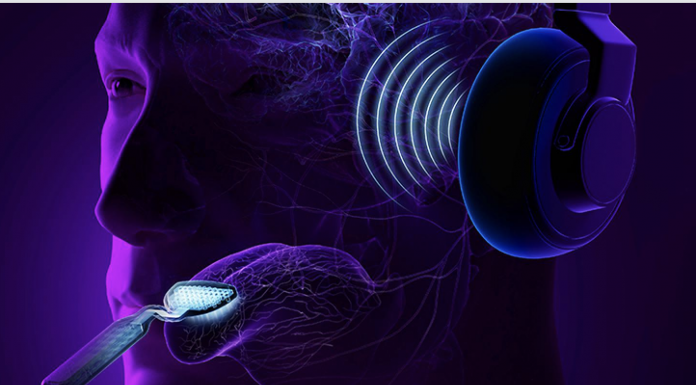
The tinnitus treatment device used in the study, now branded as Lenire®, was developed by Neuromod Devices and consists of wireless (Bluetooth®) headphones that deliver sequences of audio tones layered with wideband noise to both ears, combined with electrical stimulation pulses delivered to 32 electrodes on the tip of the tongue by a proprietary device trademarked as Tonguetip®.
The timing, intensity, and delivery of the stimuli are controlled by an easy-to-use handheld controller that each participant is trained to operate. Before using the treatment for the first time, the device is configured to the patient’s hearing profile and optimized to the patient’s sensitivity level for tongue stimulation.
For the trial, participants were instructed to use the Lenire® device for 60 minutes daily for 12 weeks. Out of 326 enrolled participants, 83.7 percent used the device at or above the minimum compliance level of 36 hours over the 12-week treatment period.
For the primary endpoints, participants achieved a statistically and clinically significant reduction in tinnitus symptom severity.
When treatment was completed, participants returned their devices and were assessed at three follow-up visits for up to 12 months.
Just more than 66 percent of participants who filled out the exit survey (n=272) affirmed they had benefited from using the device, and 77.8 percent (n=270) said they would recommend the treatment for other people with tinnitus.
Participants in the study were screened and selected based on a pre-defined list of inclusion and exclusion criteria to ensure the trial had a wide distribution sample of the tinnitus population.
To read the full research paper entitled “Bimodal neuromodulation combining sound and tongue stimulation reduces tinnitus symptoms in a large randomized clinical study,” visit the Science Translational Medicine website. Source.
ALSO ON HEADLINE HEALTH TODAY: Fauci’s New Rule; Is Your Family ‘Too Large’ For Thanksgiving Gathering? | Cops Find Endangered African Primate In San Franciso Park | Big Problem With Those Yelp Doctor Reviews
RECENTLY: