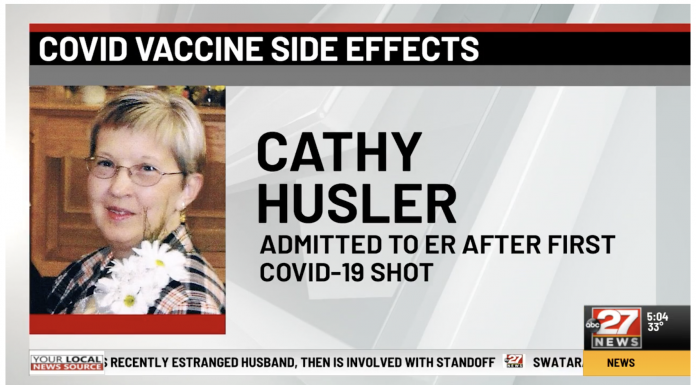
MIFFLINTOWN, Pa. (WHTM)
67-year-old Cathy Husler got her first COVID-19 shot on Feb. 3, started having chills the next day, and a day after that, had chills so bad, she went to the ER.
“My temperature was 103.8,” Husler said. She had to stay overnight at Geisinger Lewistown.
“Wanted to rule out that I didn’t have any heart issues, that I didn’t have pneumonia, did a chest x-ray, did a CT scan to rule out that I didn’t have blood clots,” Husler said.
Husler said doctors told her that her severe chills were likely related to her COVID shot.
While Husler is feeling better now, she says she’s still not 100% but says her doctor recommended she still get the second shot … Click source below to read more.
RECENTLY: 0.0064 Percent Of Vaccinations Lead To ER Observation
Does learning about vaccine reactions — including how rare severe reactions really are — make you more or less likely to get the vaccine? Comment below.
Tracking serious COVID-19 vaccine reactions
by Hannah Dineen (NEWS CENTER Maine), February 15, 2021
BRUNSWICK, Maine — Since COVID-19 vaccinations first became available, the Center for Disease Control (CDC) warned the public about common side effects of the vaccine: soreness or swelling at the injection site– or fever, chills, tiredness, and/or a headache in the days following vaccination.
Occasionally, there are more serious reactions or “adverse reactions” to the vaccine. Federal authorities are tracking those reactions through an online database.
The database is called “VAERS,” or the “Vaccine Adverse Event Reporting System.” It’s not new. VAERS was established more than two decades ago and is co-managed by the U.S. Center for Disease Control and Prevention and the Food and Drug Administration (FDA).
VAERS is used to monitor adverse reactions to all approved vaccines, most recently, the COVID-19 vaccines.
Mid Coast Hospital Chief Medical Officer Chris Bowe said, “It’s really set up so information can be obtained if there’s an unusual or unexpected reaction.”
Bowe continues, “It’s a nice system in that individuals can report it themselves. Anyone can report.”
While patients can report any reaction, health care systems are required to report the following adverse events after vaccinations occur:
- Death
- A life-threatening event
- Inpatient hospitalization or prolongation of existing hospitalization
- A persistent or significant incapacity or substantial disruption of the ability to conduct normal life functions
- A congenital anomaly/birth defect
- An important medical event that based on appropriate medical judgment may jeopardize the individual and may require medical or surgical intervention to prevent one of the outcomes listed above.
Furthermore, healthcare providers are encouraged to report to VAERS any additional clinically significant AEs following vaccination, even if they are not sure if vaccination caused the event.
The FDA and the CDC use the data to spot concerning trends and determine how often and how widely they happen … Read more.
ICYMI: OUR 5-PART SERIES FROM MICHIGAN MEDICINE ON COMMON VACCINE CONCERNS –
- Part I: “Corners Were Cut In Covid Vaccine Testing”: TRUE or FALSE?
- Part II: “The Vaccine Changes Your DNA”: TRUE or FALSE?
- Part III: “People Are Getting Seriously Ill From Vaccines”: TRUE or FALSE?
- Part IV: “The Vaccine Is Designed For Population Control”: TRUE or FALSE?
- Part V: “COVID Vaccines Have Tracers and Baby Parts”: TRUE or FALSE?