NEW DELHI, Oct 12 (Reuters) – Indian health authorities said on Wednesday they had halted all production of New Delhi-based Maiden Pharmaceuticals after a WHO report that its cough and cold syrups exported to Gambia may be linked to the deaths of dozens of children there.
The deaths of 69 children in Gambia – one of the worst such incidents involving drugs from India – have come as a blow to the industry whose exports more than doubled in the last decade to hit $24.5 billion last fiscal year … READ MORE.
Known as a “pharmacy of the world”, India supplies 45% of all generic medicines to Africa. – REUTERS
EARLIER:
By ABDOULIE JOHN Associated Press, Oct 6, 2022
BANJUL, Gambia (AP) — Gambia has launched an urgent door-to-door campaign to remove cough and cold syrups blamed for the deaths of more than 60 children from kidney injury in the tiny West African country.
Speaking to The Associated Press, the Director of Health Dr. Mustapha Bittaye confirmed the wave of child deaths from acute kidney injury, sending shockwaves across the country of 2.4 million people and around the world.
The U.N.’s World Health Organization has issued an alarm in response to the deaths, saying it had been working with Gambia’s government to investigate the cause of the cases and deaths since August.
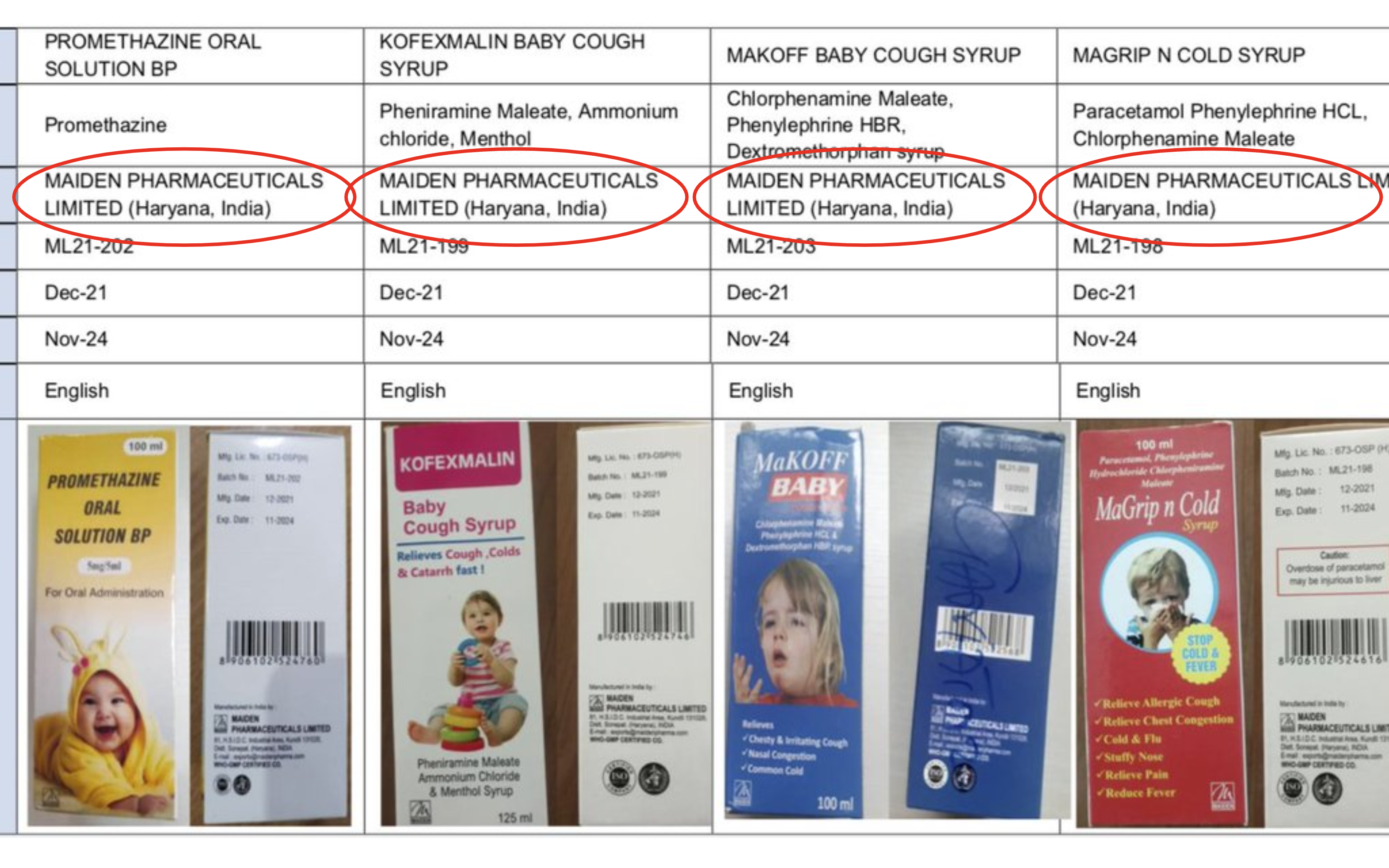
“WHO has issued a medical product alert for four contaminated medicines identified in The Gambia that have been potentially linked to acute kidney injuries and 66 deaths among children,” WHO Director-General Tedros Adhanom Ghebreyesus said Wednesday. “The loss of young lives is beyond heartbreaking for their families.”
The four medicines are cough and cold syrups produced by Maiden Pharmaceuticals Limited in India, WHO said.
While the contaminated products have so far only been detected in Gambia, they may have been distributed to other countries, WHO said. The U.N. health agency said it is pursuing investigations with the company and regulatory authorities in India.
“WHO recommends all countries detect and remove these products from circulation to prevent further harm to patients,” it said.
Teaming up with the Gambia Red Cross Society, the Ministry of Health has dispatched hundreds of young people to collect the suspect syrups through a house-to-house campaign.
Gambia’s Medical Research Council has also issued an alarm.
“Over the last week, we admitted a child with this condition (acute kidney injury) … and she has unfortunately died. We were able to confirm that she had taken one of the drugs that is suspected to be causing this, prior to her arrival at our clinic. It had been bought at a pharmacy within The Gambia,” the council said in a statement. “The drug has been identified as containing a significant amount of a toxin which damages kidneys irreversibly.”
In India, the federal regulator and the state regulator of northern Haryana state are conducting an inquiry into the contaminated medicines.
Of the 23 samples tested, four had so far been found to be contaminated and India is waiting for the analysis to be shared with it, said an Indian health official who spoke on condition of anonymity since they were not authorized to speak to the media.
Phone calls to the headquarters of Maiden Pharmaceuticals went unanswered. Neither India’s health ministry nor the federal regulator responded to queries from the AP.
___
AP journalists Jamey Keaten in Geneva and Aniruddha Ghosal in New Delhi, India, contributed.