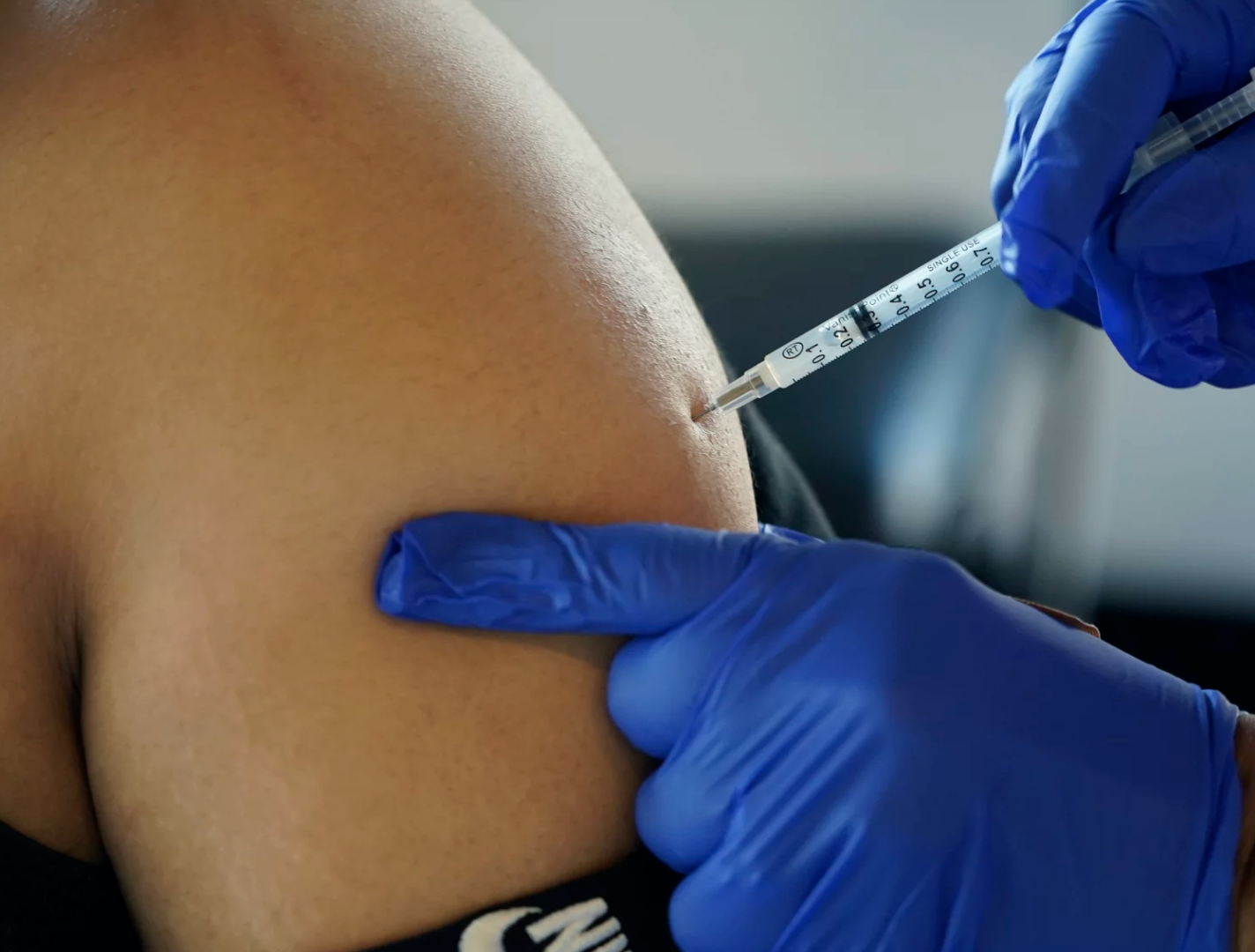
Heard on Morning Edition – The Centers for Disease Control and Prevention is recommending updated COVID boosters, for people ages 12 and older.
These newly authorized shots are reformulated versions of the Moderna and Pfizer-BioNTech COVID-19 vaccines and they’re available at pharmacies, clinics and doctors’ offices around the country.
The boosters target both the original strain of the coronavirus and the two omicron subvariants which are causing most of the current infections. Vaccine makers have scrambled to rejigger the vaccines as they’ve become less effective against new variants.
“This virus has been mutating so quickly over the past two years,” says Judith Guzman-Cottrill, an infectious disease specialist at Oregon Health & Science University. “I feel like we’ve been playing catch up and finally we have caught up,” Guzman-Cottrill says.
Pfizer’s updated booster is available for anyone 12 and older. The Moderna booster is available for anyone 18 and older … read more.
“I take my vitamins and stuff every day. So whatever keeps my immune system strong, I’m on it. I don’t really see the purpose of getting (the booster)…I never got the virus so I don’t think I really need the booster.”– Wake County, NC resident Chris Morgan. [ABC11.com]
EXPLAINER: Should you get a new COVID booster? If so, when?
By LAURAN NEERGAARD AP Medical Writer
John Wherry will wait until later in the fall to consider getting an updated COVID-19 booster. The University of Pennsylvania immunologist knows it’s too soon after his shot late this summer, especially since he’s not at high risk from the virus.
It’s the kind of calculation many Americans will face as booster shots that target currently circulating omicron strains become available to a population with widely varying risks and levels of immunity.
Here are some things to know:
HOW ARE THE NEW BOOSTERS DIFFERENT?
They’re combination or “bivalent” shots that contain half the original vaccine that’s been used since December 2020 and half protection against today’s dominant omicron versions, BA.4 and BA.5. It’s the first update to COVID-19 vaccines ever cleared by the Food and Drug Administration.
WHO’S ELIGIBLE?
Updated shots made by Pfizer and its partner BioNTech are authorized for anyone 12 and older, and rival Moderna’s version is for adults. They’re to be used as a booster for anyone who’s already had their primary vaccination series — using shots from any U.S.-cleared company — and regardless of how many boosters they’ve already gotten.
IF I JUST GOT ONE OF THE ORIGINAL BOOSTERS, SHOULD I GET THE NEW KIND RIGHT AWAY?
No. The FDA set the minimum wait time at two months. But advisers to the Centers for Disease Control and Prevention said it’s better to wait longer. Some advise at least three months, another said someone who’s not at high risk might wait as long as six months.
“If you wait a little more time, you get a better immunologic response,” said CDC adviser Dr. Sarah Long of Drexel University.
That’s because someone who recently got a booster already has more virus-fighting antibodies in their bloodstream. Antibodies gradually wane over time, and another shot too soon won’t offer much extra benefit, explained Wherry, who wasn’t involved with the government’s decision-making.
WHAT IF I RECENTLY RECOVERED FROM COVID-19?
It’s still important to get vaccinated even if you’ve already been infected — but timing matters here, too.
The CDC has long told people to defer vaccination until they’ve recovered but also that people may consider waiting for three months after recovering to get a vaccination. And several CDC advisers say waiting the three months is important, both for potentially more benefit from the shot and to reduce chances of a rare side effect, heart inflammation, that sometimes affects teen boys and young men.
HOW MUCH BENEFIT WILL THE NEW BOOSTERS OFFER?
That’s not clear, because tests of this exact recipe have only just begun in people.
The FDA cleared the new boosters based in large part on human studies of a similarly tweaked vaccine that’s just been recommended by regulators in Europe. Those tweaked shots target an earlier omicron strain, BA.1, that circulated last winter, and studies found they revved up people’s virus-fighting antibodies.
With that earlier omicron version now replaced by BA.4 and BA.5, the FDA ordered an additional tweak to the shots — and tests in mice showed they spark an equally good immune response.
There’s no way to know if antibodies produced by an omicron-matched booster might last longer than a few months. But a booster also is supposed to strengthen immune system memory, adding to protection against serious illness from the ever-mutating virus.
HOW DO WE KNOW THEY’RE SAFE?
The basic ingredients used in both omicron-targeting updated vaccines are the same. Testing by Pfizer and Moderna of their BA.1-targeted versions proved safe in human studies and CDC’s advisers concluded the additional small recipe change should be no different.
Flu vaccines are updated every year without human trials.
CAN I GET A NEW COVID-19 BOOSTER AND A FLU SHOT AT THE SAME TIME?
Yes, one in each arm.
WHAT IF I WANT TO WAIT?
People at high risk from COVID-19 are encouraged to get the new booster when they’re due. After all, BA.5 still is spreading widely and hospitalization rates in older adults have increased since spring.
Most Americans eligible for an updated booster have gone at least six months since their last shot, according to the CDC — plenty of time that another shot should trigger a good immune response.
But the original formula still offers good protection against severe illness and death, especially after that all-important first booster. So it’s not uncommon for younger and healthier people to time boosters to take advantage of a shot’s temporary jump in protection against even a mild infection, like Wherry did.
A healthy 51-year-old, Wherry said he postponed the second booster recommended for his age for seven months, until late summer — just before an international trip that he knew would increase his risk from unmasked crowds.
With the updated boosters now rolling out, he plans to evaluate in four or five months — when presumably his antibody level starts waning and he’s planning holiday gatherings, whether he’d benefit from another shot. SOURCE.
___
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Department of Science Education. The AP is solely responsible for all content.