ARS TECHNICA – An extensively drug-resistant bacterial strain is spreading in the US for the first time and causing an alarming outbreak linked to artificial tears eye drops, according to an alert released Wednesday evening from the Centers for Disease Control and Prevention.
So far, the germ has caused various infections in 55 people in 12 states, killing one and leaving others hospitalized and with permanent vision loss.
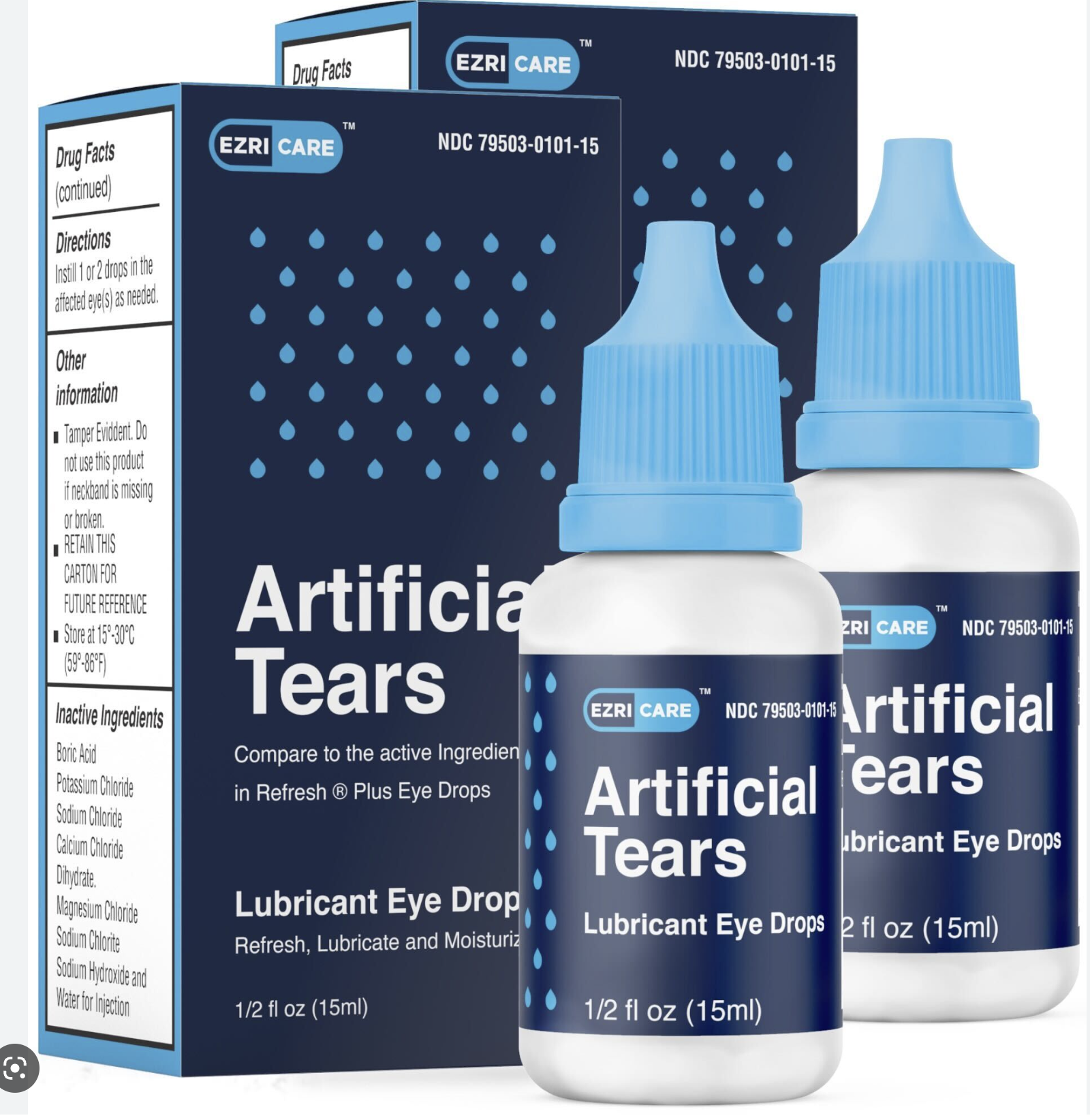
Infected patients reported using more than 10 brands of artificial tears collectively, with some patients using multiple brands. But the most common brand used among the patients was EzriCare Artificial Tears, a preservative-free product sold by Walmart, Amazon, and other retailers.
On Thursday, after this story originally published, the Food and Drug Administration posted notice of a recall of EzriCare Artificial Tears and Delsam Pharma’s Artificial Tears. The FDA the CDC recommends clinicians and patients stop buying and using the two products.
In a separate notice, the FDA further added that the products’ manufacturer, Global Pharma Healthcare Private Limited, was in violation of good manufacturing practices, including lack of appropriate microbial testing, formulating its product without an adequate preservative, and lack of proper controls concerning tamper-evident packaging.
Formidable foe
The culprit behind the outbreak is a strain of Pseudomonas aeruginosa, an extremely versatile, innately drug-resistant bacterium that lurks in the environment, particularly freshwater. It is known to cause various skin, wound, burn, lung, and systemic infections.
It most often strikes people in immune-compromised states, such as those with cystic fibrosis, and has a reputation for sparking outbreaks in health care settings, particularly among people with indwelling devices, like catheters and breathing tubes. In hospital settings, it lurks in sinks, icemakers, device washers, respiratory therapy equipment, and on soap bars.
In the current outbreak, 35 of the 55 infected patients were linked to four clusters of cases in health care facilities. Among those four health care-associated clusters, the EzriCare Artificial Tears product was the only common product among the facilities.
CDC investigators also found the outbreak P. aeruginosa strain in opened containers of EzriCare Artificial Tears bottles, which were manufactured in different lots and collected from patients in two different states.
The outbreak strain is a rare, extensively drug-resistant strain …
The cases so far occurred in 12 states: California, Colorado, Connecticut, Florida, New Jersey, New Mexico, New York, Nevada, Texas, Utah, Washington, and Wisconsin.